Pyruvate is a carbohydrate naturally found in red apples, cheese, and red wine. Scientists who have studied pyruvate believe it may accelerate fat loss by increasing cellular respiration. Some believe that calcium pyruvate may even reduce fat without exercise making it an extremely attractive “fat burner” for those people who have trouble sticking to a diet or an exercise routine. For those who can maintain a strict exercise routine but have trouble losing weight pyruvate may help them attain their goals without nervousness or jittery feelings commonly associated with most over the counter thermogenic fat burners.
Pyruvate is a substance that naturally occurs in the body. Pyruvate (because of pyruvic acid) is the foundation of the Krebs or Citric Acid Cycle. This cycle is the process (that uses pyruvate) through which the body converts glycogen to energy. Simply, it is how the body burns sugar and starch. Thus, pyruvate plays a crucial role in this conversion of food to energy. Pyruvate is the salt form of pyruvic acid. Pyruvic acid alone is chemically unstable and can cause gastrointestinal discomfort and nausea. However, when combined with calcium it becomes stabilized pyruvate. Calcium is the best stabilizing agent for pyruvate, as it attracts the least amount of water.
How Pyruvate Works: The Krebs Cycle Explained
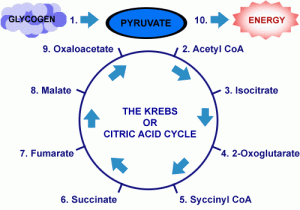
- There are ten steps to the Krebs Cycle. It takes 2 turns of the Krebs Cycle to metabolize each glycogen molecule
- The unstable bond of acetyl CoA breaks, and the two-carbon acetyl group bonds to the four-carbon oxaloacetic acid to form six-carbon citric acid.
- Two major events occur during this step: Isocitric acid loses carbon dioxide leaving a five-carbon molecule and the five-carbon compound is oxidized, reducing NAD+
- A multienzyme complex catalyzes: the removal of carbon dioxide, the oxidation of the remaining four-carbon compound, reduction of NAD+, and the attachment of CoA with a high energy bond to form succinyl CoA
- Substrate level phosphorylation occurs in a series of enzyme catalyzed reactions: the high energy bond in succinyl-CoA breaks, and some energy is conserved as CoA is displaced by a phosphate group. The phosphate group is transferred to GDP to form GTP and succinic acid. GTP donates a phosphate group to ADP to form ATP.
- Succinic acid is oxidized to fumaric acid and FAD is reduced: Two hydrogens are transferred to form FADH2(FADH2 stores less energy than NADH) The dehydrogenase that catalyzes this reaction is bound to the inner mitochondrial membrane.
- Water is added to fumaric acid which rearranges its chemical bonds to form malic acid
- Malic acid is oxidized, and NAD is reduced
- A molecule of NADH is produced
- Oxaloacetic acid is regenerated to begin the cycle again
** Note that for every turn of the Krebs Cycle:
- Two carbons enter the acetyl fragment of Acetyl CoA
- Two different carbons leave as carbon dioxide
- Coenzymes are reduced; three NADH and one FADH2 are produced
- One ATP molecule is produced by substrate level phosphorylation
- Oxaloacetic acid is regenerated
- For every glucose molecule split during glycolysis, two acetyl fragments are produced. Thus, it takes two turns of the cycle to complete the oxidation of glucose
- Reduced Coenzymes produced by the Krebs Cycle (6 NADH and 2 FADH2 per glucose) carry high energy electrons to the electron transport chain where ATP is produced by chemiosmosis. Most of the ATP output of respiration results from this oxidative phosphorylation
Summary: To put all this chemistry (which is admittedly difficult to follow) into layman’s terms, the faster the Krebs Cycle turns, the more energy (ATP) a person has, and the more calories they burn. And Pyruvate makes the Krebs Cycle run faster.
Request an appointment by texting 850-629-0345 or call our office today at 850-309-0356.

